
Acids & Bases Problem Set
Question 11: pH and buffering capacity of a mixed solution
Tutorial to help answer the questionIf equal volumes of 0.05 M NaH2PO4 and 0.05 M H3PO4 are mixed, which of the following best describes the resulting solution? (pKa's for phosphoric acid are 2.0, 6.8 and 12.0)
A. pH 2 and poorly buffered. B. pH 2 and well buffered. C. pH 6.8 and well buffered. D. pH 12 and well buffered. E. pH 6.8 and poorly buffered.
Tutorial
Recognizing the appropriate protonated and deprotonated forms of an acid with multiple ionizations| Phosphoric acid undergoes three ionizations, hence it has three pKa values
(given as 2.0, 6.8 and 12.0 in the question). Phosphoric acid has
great biological significance due to its role in DNA/RNA, energy molecules
such as ATP, protein phosphorylation, etc; therefore, it is worthwhile to spend a moment examining its ionization reactions.
Imagine the starting form as being completely protonated. Intuitively, complete protonation should occur when the [H+] is very high, ie. at a low pH. Thus, at pH 1 or less, phosphoric acid exists as >90% H3PO4. Now imagine adding NaOH to a solution of H3PO4 at pH 1. The OH- ion combines with H+ to produce water, raising pH and leaving Na+ in solution. As the pH rises towards 2.0, however, what happens to the H3PO4? Since 2.0 is the first pKa , the first proton will begin coming off. This has two consequences, one concerning the chemical form of the H3PO4 and the other concerning buffering. |
| Concerning the chemical form of H3PO4 , after the first H+ has completely
come off (which occurs when the pH has risen above 3 or so), we are left
with H2PO4 -. But since Na+ is also left by this reaction (the OH- and the
H+ having combined to produce water), we can express the
ions in solution as being Na+H2PO4 -, or just NaH2PO4 (also called
monobasic sodium phosphate).
Thus, the first ionization is written as follows:
NaOH + H3PO4 What is the equation for the third ionization? |
| As for the buffering part, one only needs to realize that, during the
transition between pH 1.0 and 3.0, a lot of the H+ used to combine with OH-
comes from H3PO4. The protons do not come from water, and the
relationship [H+] x [OH-] = 10 - 14 still holds; therefore, the pH does not change
much when NaOH is added during the 1-3 pH transition. This is not the case
between, say pH 3.0 and 5.8, when addition of NaOH does not take H+ from
phosphate, because there is no pKa value for phosphate dissociation in this
range (the second pKa skips from 2.0 all the way up to 6.8). Thus, any H+
must come from water, and the pH changes rapidly in this range.
The concept of buffering can also be shown graphically, as seen below.
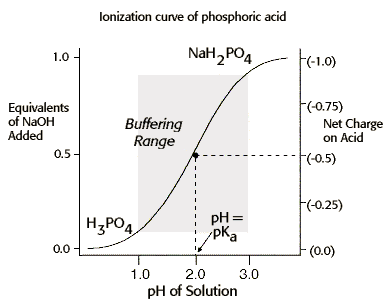
In this problem, the phosphoric acid solution will continue to resist pH changes as NaOH is added until nearly all of H3PO4 has been converted from to the H2PO4- form. At pH values >> pKa , the affinity of phosphoric acid for protons is not sufficient to bind H+ until the next pKa is reached, and at pH values << pKa , nearly all of the phosphoric acid has already bound H+ and is thus no longer available to bind additional H+. Therefore, phosphoric acid, like any other weak acid or base, is only effective as a buffer at pH values within one pH unit of its pKa . |
The University of Arizona
January 6, 1999
Contact the Development Team
http://www.biology.arizona.edu
All contents copyright © 1999. All rights reserved.