
Chemistry Tutorial Basic Chemistry for Understanding Biology
An example of biochemistry | Biology and Medicine have
enjoyed enormous benefit from a biochemical approach to life. One striking example uses the fact that spinning nuclear protons can be
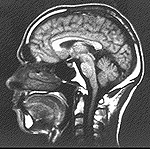
regarded as simple magnets, and interact with an external magnetic field in a way that depends on their environment. To the right is an
MRI scan of the head of a human using technology based on these principles. The technology produced high quality images of soft tissue.
|  | A review of the
basic chemistry of small molecules | The names of the elements are abbreviated. Often,
the abbreviation makes perfect sense (C for Carbon) and sometimes it does not (Na for Sodium). There is an abundance of elements on
earth and in living systems, but only 4 elements make up 99% of living organisms. These elements are Hydrogen (H), oxygen (O),
nitrogen (N), and carbon (C), and they are special in that they are 1) available and 2) suitable. |  |
Terms to know - Element
- matter composed of
atoms that all have the same atomic number (protons).
- Atom
- the smallest component of an element that still has
properties of the element, consisting of a positively charged nucleus surrounded by a charged cloud of electrons.
- "+" and "-"
charges strongly attract.
- Proton
- particle in the nucleus with a positive charge of +1 and an atomic mass number of 1
Dalton.
- Neutron
- a non-charged nuclear particle with the same mass as the proton.
- Electron
- negatively charged particle (-1) with a mass 1/1837 of that of a proton.
- Isotope
- atoms with the same number of
protons and electrons, but different numbers of neutrons.
|
Electrons determine chemical properties of elements
|
Electrons are
outside the nucleus, and determine properties of the atom. Chemical reactions
involve sharing or exchanging electrons. Electrons move about the nucleus in atomic orbitals. Absorption of energy can cause an electron
to move up to a higher energy level. The atom is stable when the outermost energy level of most atoms has eight electrons. Electrons
can be transferred carrying energy to another molecule. The H atom can carry electrons for transfering energy. Oxygen has a strong
affinity for electrons. Redox reaction transfer of electrons from one molecule (oxidized) to another (reduced). Stability can be achieved
by adding, losing, or sharing electrons.
|
|
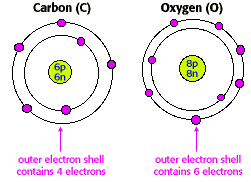
| Sharing electrons leads to the formation of covalent bonds. In the table to the right, you'll see the
bonding patterns of the primary biologically important elements. Bonds contain energy, and a require energy to be broken. Bond energy (expressed as kcal/mole) is the
energy required to break a bond. For example, an H-H bond requires 104 kcal/mole to break. | Bonding Patterns | element | number of
covalent bonds |
| H | 1 | | O | 2 | | N | 3 |
| C | 4 | | S | 5 |
|
 
   
The Biology Project
The
University of Arizona
Wednesday, June 4, 1997
Contact the Development Team
http://www.biology.arizona.edu
All contents copyright © 1997. All rights reserved.
|







