Chemistry Tutorial
The Chemistry of Water
The polarity of water|
Water has a simple molecular structure. It is composed of one oxygen atom and two hydrogen atoms.
Each hydrogen atom is covalently bonded to the oxygen via a shared pair of electrons.
Oxygen also has two unshared pairs of electrons. Thus there are 4 pairs of electrons surrounding
the oxygen atom, two pairs involved in covalent bonds with hydrogen, and two unshared pairs on
the opposite side of the oxygen atom. Oxygen is an "electronegative" or electron "loving" atom compared with hydrogen.
Water is a "polar" molecule, meaning that there is an uneven distribution of electron density.
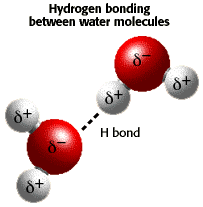
Water has a partial negative charge ( An electrostatic attraction between the partial positive charge near the hydrogen atoms and the partial negative charge near the oxygen results in the formation of a hydrogen bond as shown in the illustration. |
 |
|
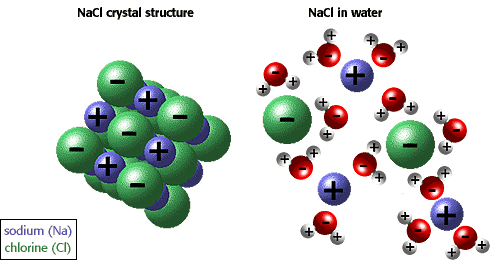
The ability of ions and other molecules to dissolve in water is due to polarity. For example,
in the illustration below sodium chloride is shown in its crystalline form and dissolved in water.
|
Many other unique properties of water are due to the hydrogen bonds. For example, ice floats because hydrogen bonds hold water molecules further apart in a solid than in a liquid, where there is one less hydrogen bond per molecule. The unique physical properties, including a high heat of vaporization, strong surface tension, high specific heat, and nearly universal solvent properties of water are also due to hydrogen bonding. The hydrophobic effect, or the exclusion of compounds containing carbon and hydrogen (non-polar compounds) is another unique property of water caused by the hydrogen bonds. The hydrophobic effect is particularly important in the formation of cell membranes. The best description is to say that water "squeezes" non-polar molecules together.
Acids and Bases, Ionization of Water
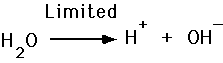
- Acid release H+
- Bases accept H+
We define the pH of a solution as the negative logarithm of the hydrogen ion concentration.
- at pH 7.0, a solution is neutral
- at lower pH (1-6), a solution is acidic
- at higher pH (8-14), a solution is basic
The University of Arizona
Wednesday, June 4, 1997
Contact the Development Team
http://www.biology.arizona.edu
All contents copyright © 1997. All rights reserved.
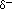 ) near the oxygen atom due
the unshared pairs of electrons, and partial positive charges (
) near the oxygen atom due
the unshared pairs of electrons, and partial positive charges (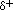 ) near
the hydrogen atoms.
) near
the hydrogen atoms.