

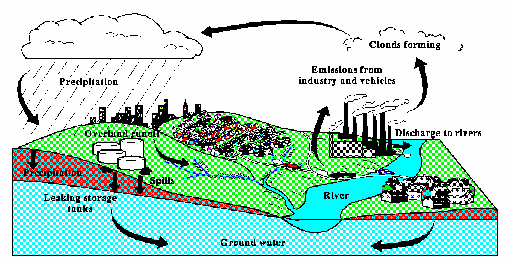
Figure 1: From U.S. G.S. Open File- Report 95-456

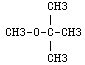
and is miscible in gasoline and is soluble in water, alcohol, and other ethers. Additional chemical properties can be reviewed from the EPA Office of Pollution Prevention and Toxics.
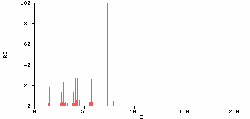 Figure 2: MTBE Mass Spectral
MTBE is the most commonly used fuel oxygenate because of its low cost, ease of production and favorable transfer and blending characteristics. It can be produced at existing refineries.
Figure 2: MTBE Mass Spectral
MTBE is the most commonly used fuel oxygenate because of its low cost, ease of production and favorable transfer and blending characteristics. It can be produced at existing refineries.

About 103 million Americans live in counties where MTBE is believed to be used (Figure 3). MTBE is on the Hazardous Air Pollutants List with 189 other chemicals to be regulated under the Air Toxics Program of the 1990 Clean Air Act Amendment. Interestingly, it was this same Clean Air Act which created the significant increase in the use of MTBE. Recently, considerable public attention has been focused on the presence of MTBE in the atmosphere , primarily the potential health risks envolved.

MTBE has been detected in groundwater supplies in eight urban areas as part of a study conducted by the United States Geological Survey and recently, MTBE has been detected in groundwater supply wells in Southern California.
MTBE is a potentially important groundwater contaminant. MTBE does not adsorb onto soil organic matter very well, thus, it moves approximately 20% faster than other gasoline constituents. Also, MTBE is persistent in groundwater because it is resistant to biodegradation under aerobic or anaerobic conditions.

Tests have been conducted to evaluate the health risks from exposure to MTBE. The USEPA has tentatively classified MTBE as a possible carcinogen and the draft drinking water lifetime health advisory level is estimated to be within the range of 20 to 200 ug/l.


The following has been added by the Electronic Desktop Project:
 Contact Us
Contact UsIf you are an educator who is using our NEXTSTEP or virtual applications in the classroom, we would especially like to hear from you. Let us know what you are doing and how it is working out. Continued support for this project will depend on its impact in science education.
If you are an educator who is interested in making use of our NEXTSTEP or virtual applications, please let us know how we can help.
 Return to the Electronic Desktop Project home page
Return to the Electronic Desktop Project home page
![]() Check out the WWW Virtual Application Catalog from the EDP
Check out the WWW Virtual Application Catalog from the EDP
 Check out the NEXTSTEP Application Catalog from the EDP
Check out the NEXTSTEP Application Catalog from the EDP
 Visit the home page for California State University, Los Angeles
Visit the home page for California State University, Los Angeles